The workhorse in both environmental and industrial heterogeneous catalysis is supported nanoparticles. Moreover, nanoparticles also play a central role in electrocatalytic applications such as fuels cells. Many catalytic materials are scarce and expensive, which makes it very important to utilize them as efficiently as possible. Nanoparticles are perfectly suited for this job, since they have extremely high catalytic surface areas per mass. Furthermore, the catalytic properties of a material often changes drastically when it is produces in the form of nanoparticles.
In our group, we work to understand catalytic nanoparticles on a fundamental level by producing model systems of size-selected nanoparticles, characterizing them, and testing them for catalytic reactions.
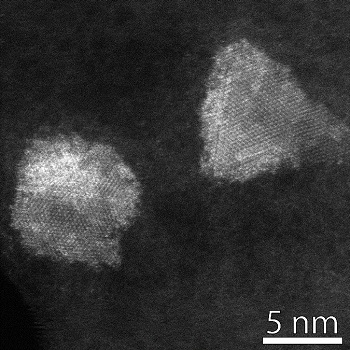
Although our research is fundamental in nature, our goals is to solve real problems in real applications, leading us to frequent collaboration with industrial partners such as Haldor Topsøe. We also collaborate internally with DTU partners, especially DTU Center for Electron Nanoscopy, which is equipped with world-class electron microscopy facilities. Below is a high-resolution STEM image of Ni-Mo-S nanoparticles where the atomic structure is clearly visible.
Going smaller
Even though traditional nanoparticle catalyst design has been incredible successful in many environmental and industrial applications, it is subject to fundamental limitations and for a number of ‘Dream Reactions’ we have no viable catalyst today.
By going smaller to nanoparticles smaller than 2 nm and all the way down to clusters with a countable number of atoms, e.g. trimers, dimers and single atoms, the catalytic properties will significantly change as compared to their nanoparticle counterparts.
At SurfCat we are using cluster sources to create model system catalysts of single atoms, dimers and slightly larger entities in the transition regime between clusters with countable atoms and large nanoparticles >2nm, to explore their catalytic properties.
Cluster sources
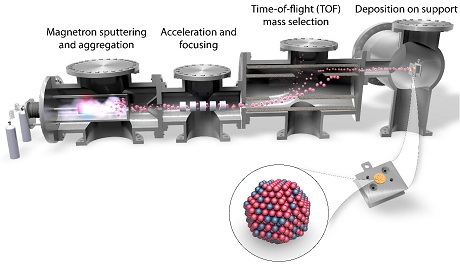
At SurfCat we have two magnetron-based cluster sources capable to producing mass-selected entities ranging from nanoparticles all the way down to single atoms. An illustration of one of the cluster sources is displayed below.
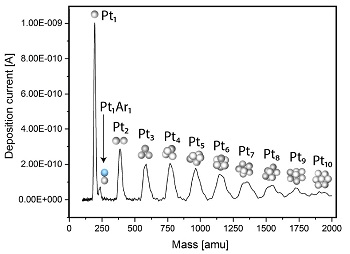
The mass filter is based on time-of-flight (TOF) orthogonal to the direction of flight, which easily allows us to select entities with a countable number of atoms. An example of a mass spectrum is displayed below where platinum entities with 1-10 atoms can clearly be resolved and selected for deposition.