The drastic drop in prices in solar and wind power is continually decreasing electricity prices. At the same time we have a chemicals and fuels industry that uses non-renewable fossil fuels as a feedsource. Our electrocatalysis groups investigates various ways to use cheap electricity and take abundant materials such as nitrogen, oxygen and CO2 and convert these into useful chemicals and fuels. At the same time we are also investigating processes where we take chemicals and convert them into electrical power or other higher value chemicals via electrochemical methods. While thermodynamics sets the theoretical limits with energy transfer between electrical power and chemicals, the practical limit is set by catalysis. Thus in this group we focus on improving catalysis to minimize losses. At the same time we need to ensure our catalysts are cheap, stable, selective and abundant enough to be scalable. While all our research is based on the same fundamental science of electrocatalysis, the reactions which we apply this knowledge to are quite varied.
Below are a list of the most prominent electrocatlytic areas we are focusing on.
- CO2 reduction to chemicals
- N2 reduction to NH3
- H2 evolution
- O2 evolution
- O2 reduction
- H2O2 reduction
- Electrosynthesis reactions from natural gas
CO2 reduction to chemicals
It has already been shown that CO2 can reduce to 18 different products, however understanding how to isolate a single product is quite challenging. Our primary focus is on the understanding of the reaction mechanisms and how we can use fundamental principles to modify catalyst to improve both activity as well as selectivity. However we also collaborate with Siemens in developing commercial scale devices, thus allowing us to cover the entire scientific spectrum from fundamentals to applications.
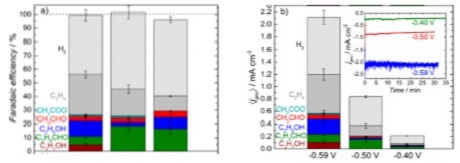
N2 reduction to NH3
With air being 80% N2 there is ample opportunity to react this electrochemically to produce ammonia for fertilizers. Currently all industrial ammonia production is produced using the high temperature, high pressure Haber-Bosch process, but this approach is capital intensive and can not be decentralized, whereas electrochemical ammonia production can be. Since nitrogen has a triple bond, it is quite stable, thus this reaction is very challenging. However we are making quite exciting breakthroughs and this is a new field so the potential for breakthroughs is quite high.
H2 evolution
With H2 consisting of 2 protons and 2 electrons it is a relatively easy molecule to produce. However the best catalyst Pt is quite expensive and we are looking at ways to find cheaper catalysts. At the same time, hydrogen’s simplicity makes it an easy molecule to understand fundamental principles in electrocatalysis, thus there is significant basic research done using this reaction primarily in relation to other reactions.
O2 evolution
CO2 to chemicals, N2 to ammonia, and H2 evolution are all reduction reactions and need a corresponding oxidation reaction for the mass balance reasons. All of these reactions, thus need corresponding oxygen evolution from water. Thus this is an essential reaction, but also a difficult reaction because two of the intermediates are linked via a scaling relationship, which means optimizing for one intermediate, deoptimizes the other intermediates. We are currently looking at creative ways to resolve this.
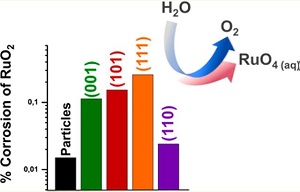
O2 evolution: We have show corrosion effects related to the highly active RuO2 electrocatalyst. Roy, et al. ACS Energy Letters 2018 3 (9), 2045-2051 DOI: 10.1021/acsenergylett.8b01178
O2 reduction
Oxygen reduction to water is the opposite reaction to oxygen evolution and is important for fuel cells, thus we have a strong focus in this field. Our primary goal is to increase the intrinsic activity, which will lower the amount of precious metal cat
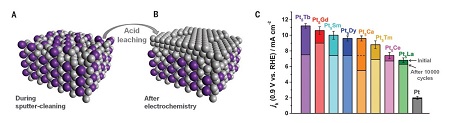
O2 reduction: Activity of Pt-Lanthanides for O2 reduction. Escudero-Escribano, M, et al. Science 01 Apr 2016: Vol. 352, Issue 6281, pp. 73-76 DOI: 10.1126/science.aad8892
H2O2 evolution
While oxygen reduction by a 4 electron step process yield water, oxygen reduction by a 2 electron step yields hydrogen peroxide. With H2O2 being an environmentally safe disinfectant there is a large potential to use this as a cleaning agent. While our focus is improving catalysis, we do have a recent spin-out company HPNow that focuses on applying this technology commercially.
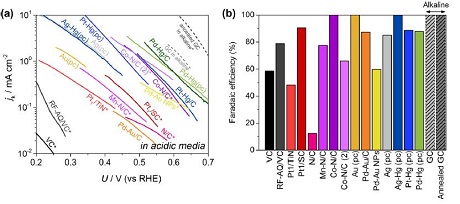
H2O2: See our recent review on state of the art H2O2 catalysis. Yang et al. ACS Catalysis 2018 8 (5), 4064-4081 DOI: 10.1021/acscatal.8b00217
Electrosynthesis reactions
A serious immediate problem is that for oil wells that are at isolated locations it is more economic to burn/flare accompanying natural gas than to actually sell it. Thus this is a completely unnecessary contribution to climate change. In our laboratories we investigate converting natural gas consituents (methane and propene) into useful products via our electrocatalysts such as methanol, acrolein, and acrylic acid to prevent useless flaring by the oil industry.
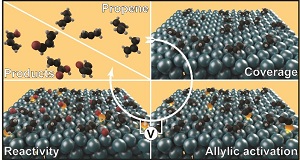
Electrosynthesis: Propene oxidation on Pd. Winiwarter et al. Submitted
As part of our work with collaborate with many groups both internally and externally. Some of our largest external collaborators are Stanford and MIT, whereas internally we collaborate with the newly formed Theo-Cat section at DTU physics.